Individual Water Molecules Bind to Each Other Through
Each H 2 O can bind to a maximum of four neighbors through these so-called hydrogen bonds. 3 How many molecules of oxygen can each molecule of hemoglobin carry quizlet.

Hydrogen Bonds Make Water Sticky Manoa Hawaii Edu Exploringourfluidearth
D All water molecules are neutral.

. Ionic covalent hydrogen 24What is the theory of Endosymbiosis. These interactions fall into two main categories. Due to the polar nature of water the molecules of water are not as tightly packed in solid water ice as they are in liquid water.
As water freezes it takes up more hydrogen from the atmosphere causing it to have a greater buoyancy. Mitochondria and chloroplasts are derived from bacteria and algae organelles funtion in tandem. A polar solute on the other hand is not attracted by the non polar solvent and so the bonds are not broken.
Individual water molecules bind to each other through. 5 How many globin molecules are found in a single hemoglobin molecule. A water molecule is formed by covalent bonds between an oxygen atom and two hydrogen atoms.
Each subunit surrounds a central heme group that contains iron and binds one oxygen molecule allowing each hemoglobin molecule to bind four oxygen molecules. Water molecules readily form hydrogen bonds that can compete with ligands for sites on the protein surface. Water molecules stick to each other through hydrogen bonds.
1 The interaction of neighboring parts of the polypeptide chain may restrict the access of water molecules to the proteins ligand-binding sites. Question 14 30 seconds Q. Each myoglobin molecule is capable of binding one oxygen becausemyoglobin contains one heme per molecule.
Binding strength of molecules that bind elsewhere in part by changing the proteins structure. Dror et al Nature 2013 You can simulate how a molecule binds to a protein and in turn what effect binding will have on protein structure. In effect a ligand binding site can be kept dry because it is energetically unfavorable for individual water molecules to break away from this network as they must do to reach into a crevice on a.
A non polar solute will be dissolved by a non polar solvent because the two are attracted to one another and the bonds binding the molecules of the non polar solute are broken. D when there is the same number of water molecules as dissolved sugar molecules. Why does ice float.
4 How many oxygen molecules can bind to each one of these molecules. Hydrogen bonds form between water molecules. Model Worksheet Teacher Key.
Yes Water molecules attract each other through hydrogen bonding Are the molecules in water close to each other. Water molecules are attracted to each other because. What happens when one molecule of o2 binds to hemoglobin.
Generally lipid molecules are not large enough to be considered macromolecules. Nicolle Rager Fuller National Science Foundation. B Each water molecule has the same number of protons as electrons.
22Individual water molecules bind to each other through covalent bonds ionic bonds hydrogen bonds 23What kind is it when one atom takes an electron from another atom. C when the dissolved sugar molecules are evenly distributed throughout the solution. A when molecules of sugar stop moving.
Why do nonpolar substances dissolve in nonpolar solvents. This is because the nucleus of the oxygen atom is more attractive to electrons than the nuclei of the hydrogen atom. Correctly bound molecules fit perfectly like puzzle pieces.
A They are all positively charged. Correct binding results in lower energy than incorrect binding. Answer choices As water freezes it expands and its density decreases.
As a result oxygenated. The molecules in water are closer to each other than those of water vapor but. The molecules have sensors that check for incorrect bindings.
Although short-lived and much weaker than the covalent variety hydrogen bonds contribute significantly to water chemistry because they are extremely abundant in H 2 O. C The positive area of one water molecule is attracted to the negative area of another. ACovalent bonds BIonic bonds CHydrogen bonds DHydrophobic bonds ENon-polar bonds.
This framework leaves ample room for guest water molecules to move in and out of its pores. Therefore in a given volume of ice there are fewer water molecules than in the same given volume of water. Because of the strong tendency of water molecules to form waterwater hydrogen bonds however water molecules exist in a large hydrogen-bonded network see Panel 2-2 pp.
The bond between hydrogen and oxygen involves unequal sharing of electron it is a polar covalent bond. B when water and sugar molecules are moving at the same speed. Water cools very rapidly.
Lipids are a diverse group of hydrophobic molecules that interact with each other through hydrophobic interactions rather than covalent bonds and are not considered true polymers. The two molecules send signals to each other. How does a molecule bind to its correct partner and avoid incorrect interactions.
MOFs are scaffold-like molecules constructed of inorganic metal clusters connected to each other by organic linker molecules. As mentioned previously all water-soluble signal molecules including neurotransmitters and all signal proteins bind to specific receptor proteins on. We then used that information to alter the molecule such that it has a different effect.

Hydrogen Bonds In Water Article Khan Academy
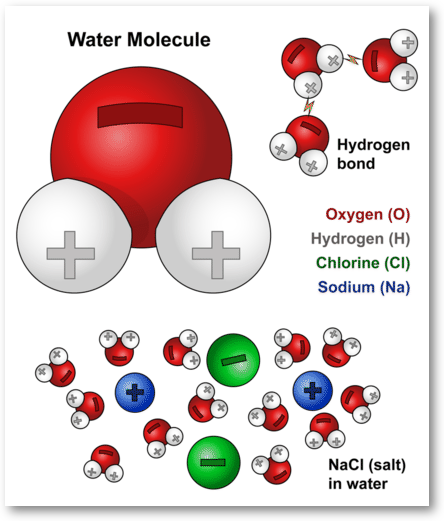
Water Molecules And Their Interaction With Salt U S Geological Survey
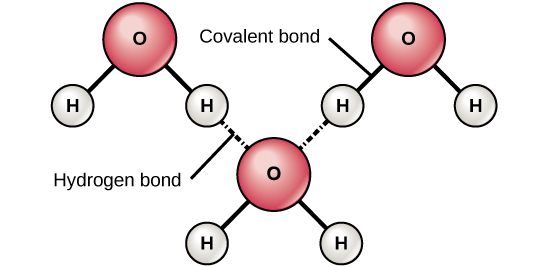
Weaker Bonds In Biology Biology For Non Majors I

Hydrogen Bond Ck 12 Foundation

Chemistry Ii Water And Organic Molecules

Soil Water From Molecular Structure To Behavior Learn Science At Scitable

Comparison Of Water With Other Liquids Manoa Hawaii Edu Exploringourfluidearth

Hydrogen Bonding Chemistry For Non Majors
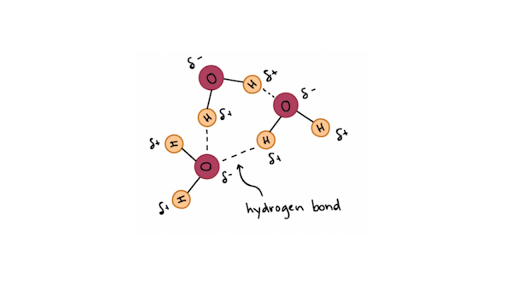
Hydrogen Bonds In Water Article Khan Academy
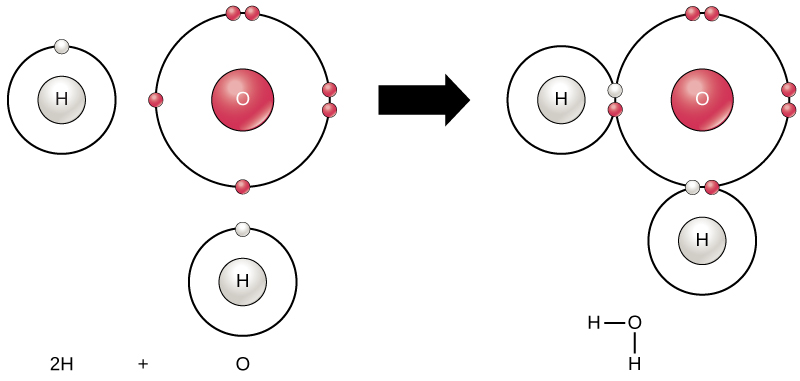
Chemical Reactions And Molecules Biology For Majors I

Organic Molecules Microbiology
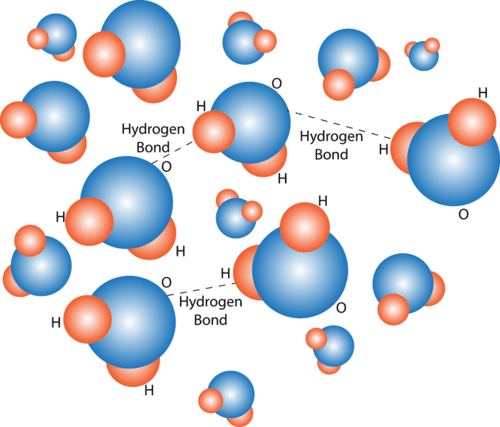
Biochemical Properties Of Water Advanced Read Biology Ck 12 Foundation
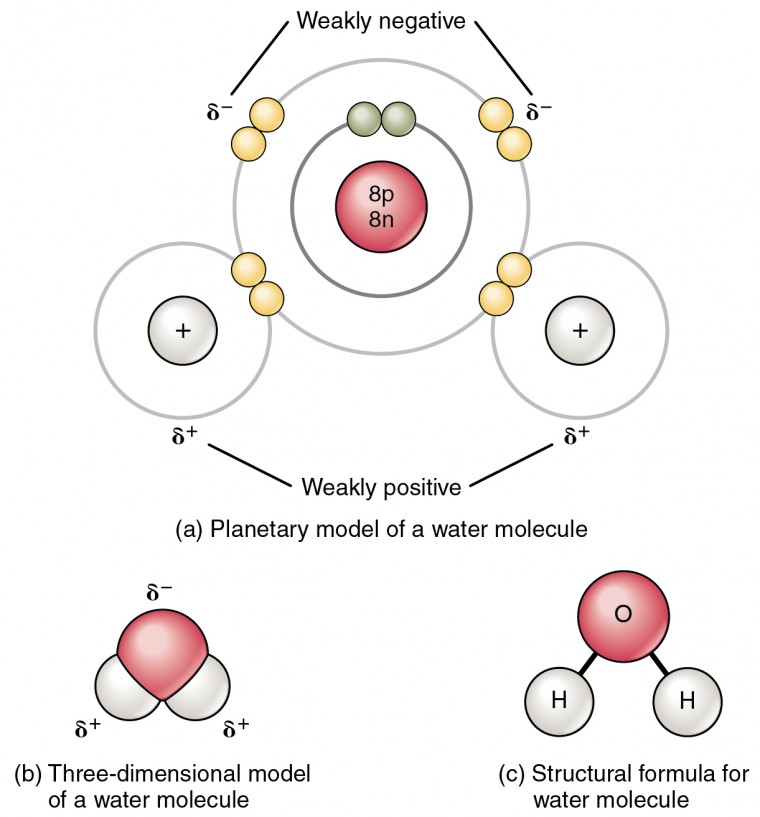
Chemical Bonds Anatomy And Physiology I

Molecules Anatomy And Physiology I

Structure Of Water Ck 12 Foundation

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

The Chemistry Of Water Water Molecules Nsf National Science Foundation
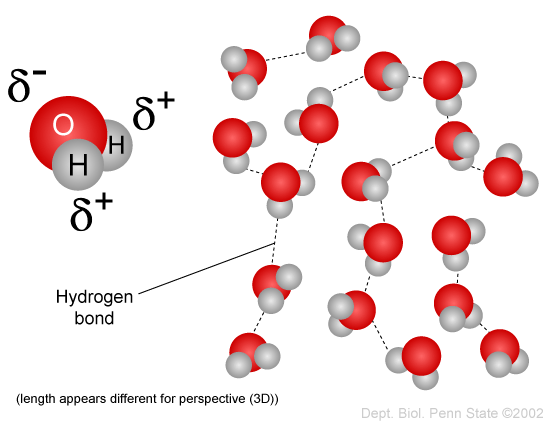
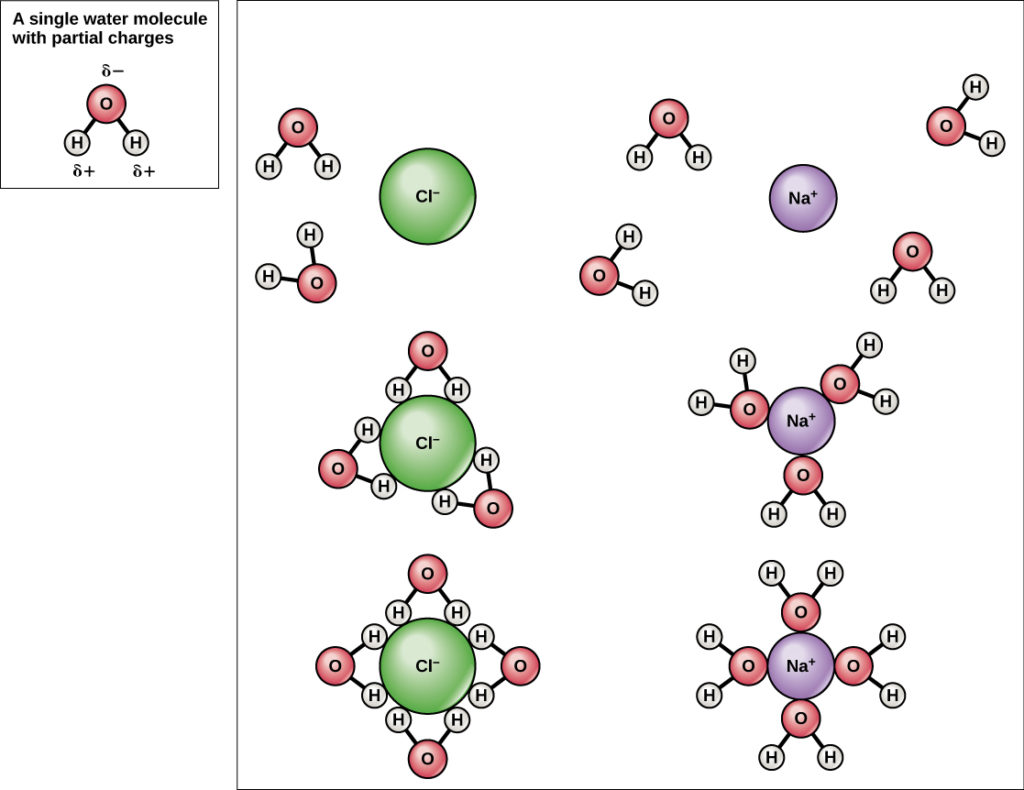
Comments
Post a Comment